Evolution and the Second Law of Thermodynamics
Hello, my name is Richard Kent. Today, I want to delve into the significance of the second law of thermodynamics. If you haven’t heard about it before, it’s crucial to grasp its implications. The second law of thermodynamics revolves around the concept of entropy. Allow me to simplify entropy before delving into its complexities.
Understanding Entropy:
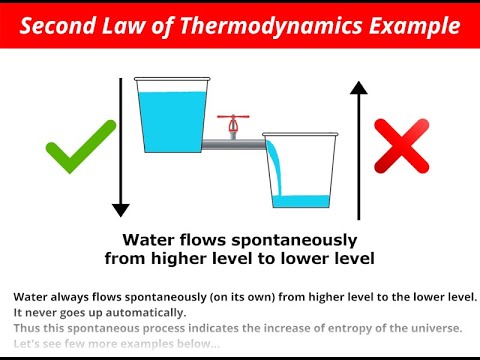
Entropy essentially refers to the increasing disorder within a system. Let’s consider a simple scenario: imagine holding a hot cup of tea. After half an hour, the tea cools down, and the room temperature slightly rises. This phenomenon exemplifies entropy at work—the system moves towards disorder.
Now, let’s scale up and contemplate the Sun’s dynamics. I frequently reference the Sun because it serves as the primary energy source in our solar system. Within the Sun, a continuous process occurs where hydrogen is converted into helium, emitting light, heat, and energy. Consequently, the Sun gradually diminishes in size over time. This continual decrease in available energy within the Sun is a manifestation of entropy, eventually leading to the hypothetical scenario of heat death.
Expanding Perspectives:
Entropy isn’t confined to heat and energy; it extends to all systems. Consider my cluttered study as another example. Every few months, the disorder in my study increases, necessitating a thorough tidying session. This phenomenon mirrors the concept of entropy—disorder tends to escalate within systems.
Moreover, entropy manifests within our bodies as we age. Personal anecdotes aside, entropy dictates that our bodily systems gradually decline over time. Despite our best efforts, aging is an inevitable consequence of entropy’s influence.
Contrasting Views:
Now, let’s contrast the principles of entropy with evolutionary teachings. Evolution posits that approximately 13.8 billion years ago, the universe originated from a chance occurrence—the Big Bang. This event supposedly led to the emergence of highly ordered systems, such as our planet Earth.
Consider the intricacies of our solar system—the precise distance between Earth and the Sun, our orbital velocity, and numerous other factors. Evolutionists attribute these complexities to random chance, dismissing the meticulous design ascribed to a higher intelligence.
Challenging Assumptions:
However, such viewpoints defy the principles of thermodynamics. The first and second laws of thermodynamics establish that systems tend toward disorder, contradicting the notion of increasing order resulting from chaotic events like explosions.
Have you ever witnessed an explosion yielding a structured outcome? The answer is likely no. Explosions typically induce chaos, not organization. Therefore, attributing the intricacies of the universe to random chance undermines fundamental scientific principles.
In Conclusion:
In essence, evolution fails to align with the principles of thermodynamics. As I conclude, I’d like to reiterate the profound words: “In the beginning, God created the heavens and the earth.” Thank you for your attention, and may God bless you abundantly.